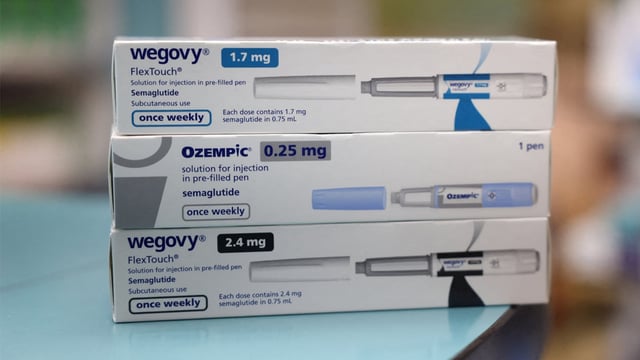
Overview
- Under Disposición 5842/2025, ANMAT prohibited the use, distribution and sale of the falsified “Ozempíc® Semaglutida Tablets” across Argentina.
- Authorities verified that Pharma Argentina S.A. and MD Pharma lack ANMAT registration or authorization to manufacture any form of semaglutide.
- Novo Nordisk’s technical director provided a sample and confirmed that Ozempic is only approved as an injectable solution, not in tablet form.
- ANMAT warned that the counterfeit product carries unknown composition and risks, and urged health professionals and consumers to check traceability labels.
- The case has been sent to the Unidad Fiscal Especializada en Ciberdelincuencia and criminal courts, which have opened a judicial inquiry into the illicit online sales.