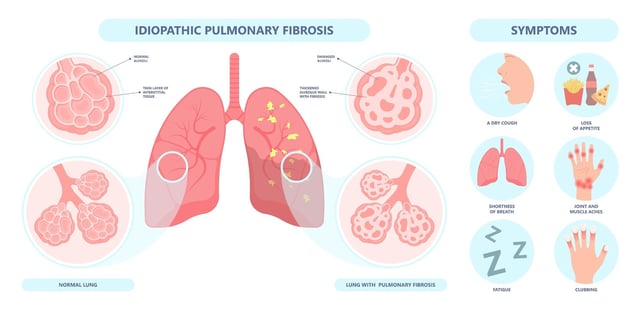
Overview
- The randomized Phase IIa GENESIS-IPF trial enrolled 71 patients across 22 sites in China, administering Rentosertib or placebo over 12 weeks.
- Participants receiving the highest 60 mg once-daily dose saw a mean forced vital capacity increase of 98.4 mL, contrasting with a 20.3 mL decline in the placebo group.
- Treatment-emergent adverse events were mostly mild to moderate with rare serious events, though several patients experienced liver injury requiring monitoring.
- Insilico Medicine’s Pharma.AI platform designed Rentosertib by targeting TNIK kinase and compressed the drug discovery timeline to 12–18 months.
- The company plans larger, longer trials in China and the US to confirm Rentosertib’s therapeutic benefit and safety before seeking regulatory approval.