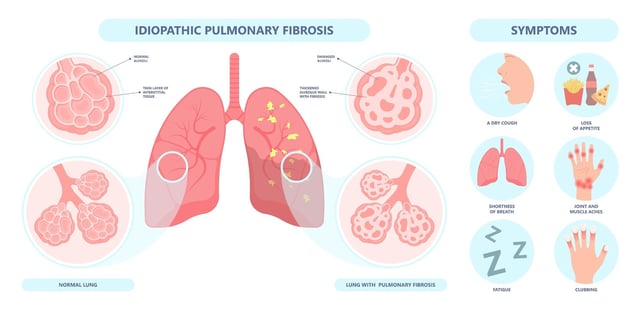
Overview
- The randomized, double-blind Phase IIa trial enrolled 71 idiopathic pulmonary fibrosis patients across 22 sites in China to evaluate Rentosertib over 12 weeks.
- Participants receiving the highest dose of 60 mg once daily saw a mean forced vital capacity increase of 98.4 mL compared with a 20.3 mL decline in the placebo group.
- Most treatment-emergent adverse events were mild to moderate, although several patients developed liver injury that will be closely monitored in follow-on studies.
- Insilico’s generative AI platform, Pharma.AI, accelerated drug discovery by nominating Rentosertib within 12–18 months, far shorter than traditional timelines.
- Following publication in Nature Medicine, the company is engaging regulators and planning larger-scale, longer-duration trials to confirm Rentosertib’s therapeutic benefit and safety.