Overview
- The guidelines ban social media influencer promotions and targeted cosmetic advertising to under-18s and impose a mandatory seven-day cooling-off period for minors.
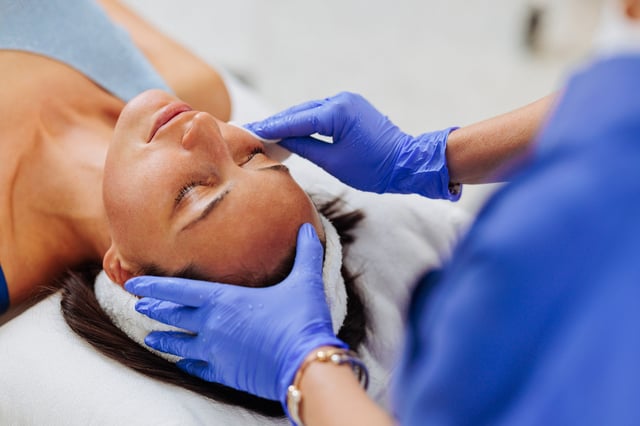
- From September nurses must complete at least 12 months of full-time clinical experience before performing non-surgical injectable procedures.
- Practitioners are required to conduct detailed patient evaluations with realistic-expectation assessments and informed-consent checklists, making ultra-short telehealth consultations unviable.
- Registered health practitioners must disclose any financial interests that could influence their advice and provide written details of products used and aftercare arrangements.
- The Therapeutic Goods Administration has issued around 100 guidance letters for unlawful advertising and plans further compliance actions ahead of the guideline rollout.