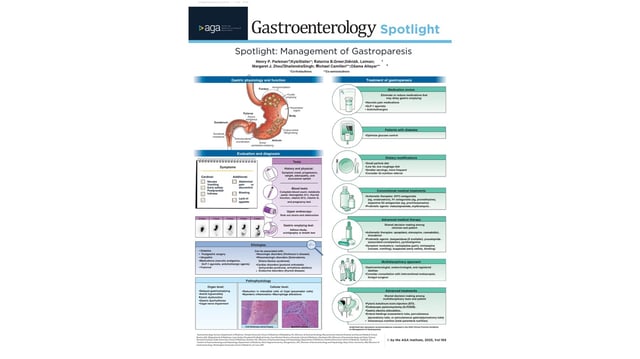
Overview
- The guidance names metoclopramide or erythromycin as appropriate initial pharmacologic options.
- It suggests against first-line use of domperidone, prucalopride, aprepitant, nortriptyline, and buspirone, and advises reserving cannabidiol for clinical trials.
- For refractory disease, it advises against routine botulinum toxin injection, G-POEM, and gastric electrical stimulation, and makes no recommendation on surgical pyloric procedures.
- All recommendations are conditional and intended to facilitate shared decision-making rather than mandate care, with “suggested against” not precluding use in select patients.
- The panel incorporated public comment and calls for research to refine disease definitions and expand treatments, with the guideline published in Gastroenterology.