Overview
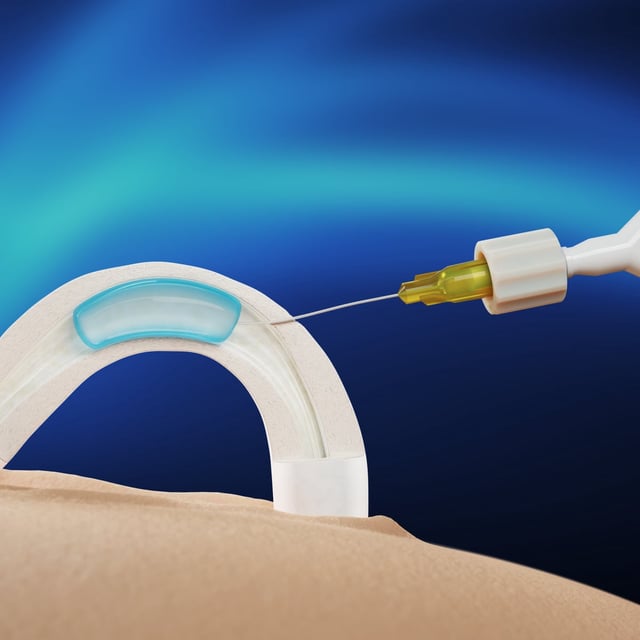
- The Adam implant, a water-soluble hydrogel, blocks sperm release by being inserted into the vas deferens through a minimally invasive 10-minute procedure.
- Phase 1 trials confirmed the implant’s effectiveness in preventing sperm release for up to 24 months, with no serious adverse events reported.
- Contraline plans to initiate a phase 2 clinical trial in Australia later this year, involving 30 to 50 participants, following regulatory approval.
- The implant is designed to dissolve over time, theoretically restoring fertility, but experts stress that data on reversibility and removal safety has not yet been published.
- The Adam Study will be presented at the American Urological Association meeting on April 26, marking a significant step in male contraceptive innovation.