Overview
- Phase 1 trial results reveal the Adam implant effectively blocks sperm release for 24 months in participants without serious adverse events.
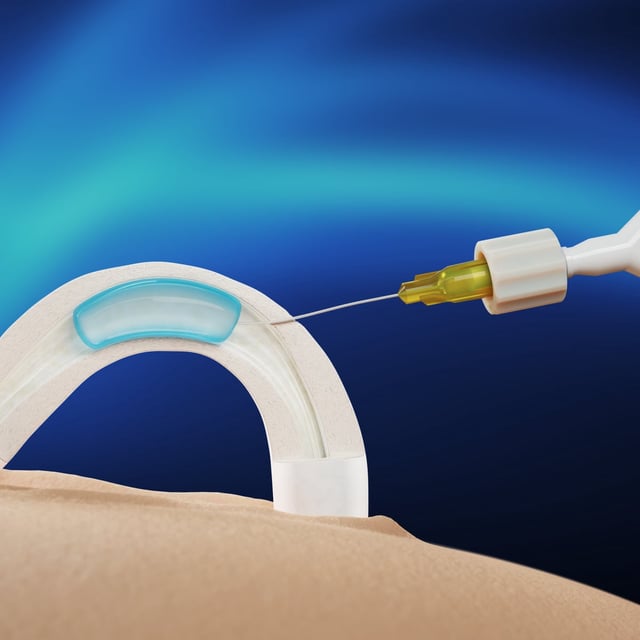
- The implant uses a water-soluble hydrogel designed to dissolve naturally, offering a reversible alternative to condoms and vasectomies.
- The 10-minute minimally invasive procedure, performed under local anaesthetic, makes the implant outpatient-friendly.
- Contraline has received regulatory approval to begin a phase 2 clinical trial in Australia later in 2025 with 30–50 participants.
- Experts highlight the need for more data on the implant’s reversibility, removal process, and long-term effects.