Overview
- Jim O’Neill urged manufacturers on Oct. 6 to develop single‑antigen measles, mumps and rubella shots, echoing President Trump’s late‑September call.
- HHS confirmed O’Neill agrees with the president, asserting separate shots could reduce side effects and maximize parental choice.
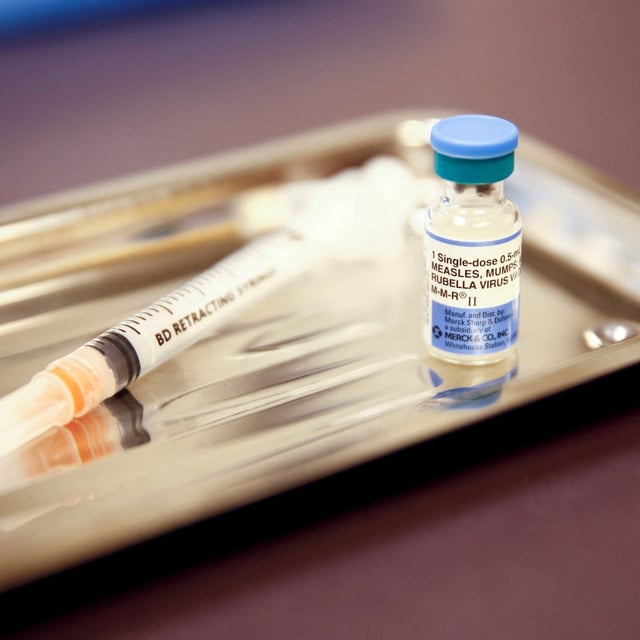
- Merck said there is no evidence separating MMR benefits patients and warned more injections could lead to delayed or missed immunizations.
- Public‑health leaders, including the CDC and the American Academy of Pediatrics, continue to recommend the combined MMR as safe, effective and easier to complete on time.
- There are no approved U.S. monovalent MMR components, development would take years with significant ethical and regulatory hurdles, and the debate comes as the U.S. has reported 1,544 measles cases in 2025.