Overview
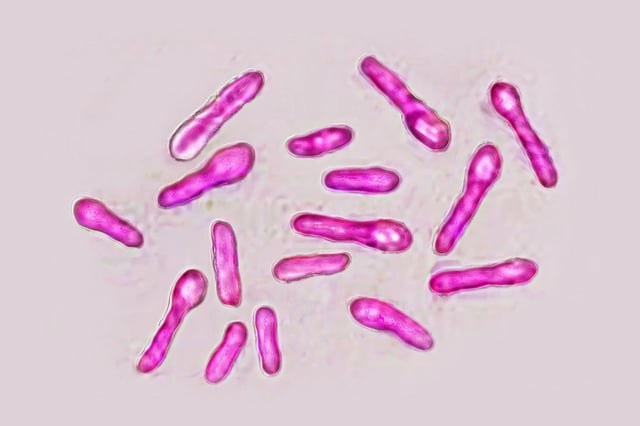
- The UK Health Security Agency and MHRA have confirmed investigations into 28 suspected botulism cases following cosmetic injections in recent weeks.
- Preliminary findings show no contamination of licensed stocks, suggesting victims received counterfeit or unauthorised botulinum toxin products.
- Reported symptoms include severe drooping eyelids, double vision, swallowing difficulties, slurred speech and profound lethargy emerging up to four weeks post-injection.
- Most practitioners tied to the outbreak have stopped offering cosmetic injections as the number of new reports begins to decline.
- Regulators and experts warn consumers to only seek treatments from qualified professionals who follow strict hygiene protocols and provide full product transparency.