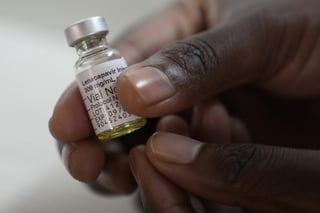
Pre-exposure prophylaxis (PrEP) is the use of medications to prevent the spread of disease in people who have not yet been exposed to a disease-causing agent, usually a virus. The term typically refers to the use of antiviral drugs as a strategy for the prevention of HIV/AIDS. PrEP is one of a number of HIV prevention strategies for people who are HIV negative but who have a higher risk of acquiring HIV, including sexually active adults at increased risk of contracting HIV, people who engage in intravenous drug use (see drug injection), and serodiscordant sexually active couples. When used as directed, PrEP has been shown to be highly effective, reducing the risk of acquiring HIV by up to 99%. As of 2019[update], the World Health Organization (WHO) recommends two drug combinations for the use as PrEP for HIV/AIDS: the combination of tenofovir disoproxil and emtricitabine (Truvada), or the combination of tenofovir disoproxil and lamivudine (Cimduo). In October 2019, the US Food and Drug Administration (FDA) approved the combination of emtricitabine and tenofovir alafenamide (Descovy) to be used as PrEP in addition to Truvada, which provides similar levels of protection. In December 2021, the FDA approved cabotegravir (Apretude), which is an injectable form of PrEP manufactured by ViiV, a pharmaceutical company that specializes in HIV drugs. Regulators believe it will improve medication adherence because it only has to be taken once every two months, and will also widen adoption as it eliminates the need to hide pills or pharmacy visits for discretion. From Wikipedia