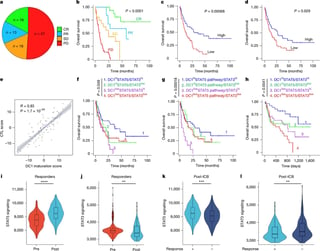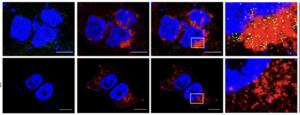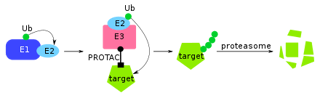

Enrolling 624 previously treated patients at the American Society of Clinical Oncology meeting in Chicago, the trial delivered a more than three-month progression-free survival advantage over Faslodex, prompting Arvinas to cancel two other planned late-stage studies.